|
|
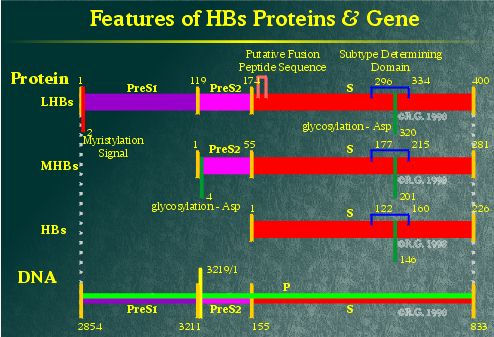
Hepatitis
B Surface Proteins
Diagrammed below are the predicted folding patterns
of the various hepatitis B virus (HBV) surface proteins.
Within the HBV genome, the
region encoding the HBV surface proteins contains three in-frame start
sites which share a common termination codon. Because of this, the various
HBV surface proteins are all related to each other by a shared region
known as the S-domain.
Small
HBsAg | Subtypes | Middle
HBsAg | Large HBsAg
Small
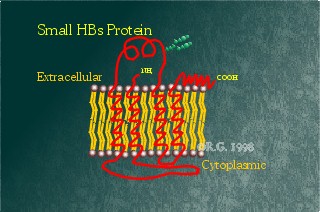
Hepatitis B Surface Antigen (HBsAg or SHBsAg)
This protein is the smallest of the hepatitis B surface proteins,
containing solely the S-domain. Historically, it also has been referred
to as the Australia antigen (Au antigen). It is
highly hydrophobic, containing four-transmembrane spanning regions. The
HBsAg contains a high number of cysteines,14 all together, each of which
is cross-linked to one another. It also may be glycosylated at Asp146. The
two forms of this protein are commonly seen on gels run on HBsAg
particles purified from carrier serum. This protein is the prime constituent
of all hepatitis B particle forms. As such, this
protein appears to be manufactured by the virus in high quantities. It also
contains a highly antigenic epitope. Analysis of this epitope allows for
the subtyping of HBV carriers.
Computer modelling implicates
helices 3 and 4 as transmembrane spanning regions inserted postranslationally
into the ER membrane. These two helices are thought to be the site of
multimerization. This has been supported by observations which show truncated
versions of the HBsAg missing in these helices. These helices are unable
to form particles and remain in the ER.
Infected cells in the early
stages produce this protein in the greatest quantities. The titre of the
resulting non-infectious HBsAg particles found in a carrier's serum can
be as high as 200ug/mL. Expression of the HBsAg appears to be inducible
by stress in the endoplasmic reticulum typically due to the presence of
high amount of LHBsAg.
Despite the high antigenicity
and prevalence of these particles, the immune system appears basically
oblivious to their presence. Studies, on T-lymphocyte-derived soluble
factors in the maintenance of HBV infection, have shown that HBsAg of
T-cell origin appears to suppress HBsAg-antibody production in other T-cells.
Suppression is antigen specific for HBsAg. Immune suppression of antibodies
against the various components of HBV favours persistence found in the
chronic HBV carrier state.
The S promoter lies within
the preS region. Mutations in this region result in lowered HBsAg production.
Reduced production of the HBsAg appears to lead to intracellular retention
of the virus. It also causes viral misassembly.
Subtypes
Subtypes of SHBsAg were originally defined by antibody recognition.
Antigenic domains present on all known HBs isolates were classified as determinant
a. The four other major subtypes are d or y and w
or r. These two sets are paired and the members of each pair are
mutually exclusive. Determinant d has a lysine at residue 122 while
y has an arginine. Similarly, determinant w has a lysine at
residue 160 while r has an arginine.
Recently, other determinants
have been found which contain antigenic epitopes unrecognizable by antibodies
against the above-mentioned subtypes. Because some antibodies are sub-type
specific, it leads to the question: Does vaccination using HBs particles
immunize a person against all HBV strains? The answer is "Yes",
so far. However, in the more recent years, escape mutants have been found,
showing a need for an improved vaccine or treatment.
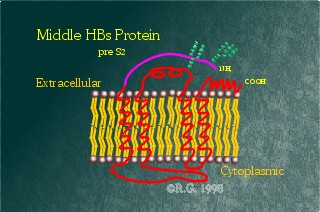
Middle
Hepatitis B Surface Antigen (MHBsAg)
This intermediate or middle-sized HBV surface protein contains
an additional 55 amino-acid domain known as Pre-S2. This domain is hydrophilic
and appears to reside extracellularly. The Pre-S2 domain also contains an
additional glycosylation site at Asp4. It appears that this site is always
glycosylated, but the glycosylation site on the S-domain is only glycosylated
at times, resulting in either a fully or partially glycosylated form of
this protein.
Some have proposed that this
protein is involved in HBV attachment and entry into the liver. However,
in a study involving genetic analysis of HBV in patients with fulminant
hepatitis, the pre-S2 start codon carried a double mutation, preventing
expression of the corresponding protein. As such, it appears pre-S2 is
not required for HBV infectivity nor viral particle morphogenesis. This
likely excludes the middle HBsAg from being the HBV binding protein, though
it may contribute to viral attachment as a secondary mechanism.
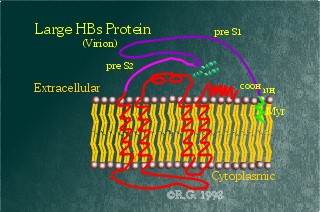
Large
Hepatitis B Surface Antigen (LHBsAg)
This protein is the largest of the HBV surface proteins, containing
the Pre-S1 domain as well as the Pre-S2 and S domains. The Pre-S1 domain's
sequence appears to be highly variable among infected patients, suggesting
that this may be the HBV protein involved in liver attachment. The Pre-S1
domain contains no additional glycosylation sites, but contains a myristylation
signal at its N-terminus, anchoring the N-terminus to the membrane.
There are two proposed different
folding patterns for this protein: one found on the cell surface and in
mature virions, the other found on the surface of the endoplasmic reticulum
(ER). The predicted folding patterns are based on protease and antibody
studies. In the related duck hepatitis B virus, the LHBsAg has also been
shown to have dual topology. It appears that both the Pre-S1 and Pre-S2
domains remain cytoplasmic when the LHBsAg is in the ER. As such, the
Pre-S2 domain remains unglycosylated whereas the Pre-S1 domains becomes
myristylated. When, where, and how the PreS domains are translocated across
the membrane are still under debate. However, a model has been proposed
for the duck hepatitis B viral model. The model predicts that the PreS
domains are translocated through an aqueous pore in the virus envelope.
This pore is likely formed by the oligomerization of the transmembrane
spanning regions in the S-domain
Overexpression of the LHBsAg
alone results in ER retention of the protein. However, it was first suggested
that ER retention was due to a cytosolic factor binding the LHBsAg as
a transmembrane protein. However, more recent evidence shows formation
of intracellular particles of LHBsAg in the lumen of the ER. Retention
appears to be due to the binding of LHBsAg to calnexin.
This protein is believed by
most to be the one responsible for mediating viral attachment onto its
host cells. However, the receptor for HBV has not been isolated.

|
|
References
Bancroft, W.H., Mundon,
F.K. and Russell, P.K. 1972. Detection of Additional Antigenic Determinants
of Hepatitis B Antigen. J Immuno; 109: 985-992.
Bock, C.T., Tillmann, H.L.,
Maschek, H.J., Manns, M.P., and Trautwein, C. A PreS Mutation Isolated from
a Patient with Chronic Hepatitis B Infection Leads to Virus Retention and Misassembly.
Gastroenterology; 113(6): 1976-1982.
Bruss, V. and Ganem, D.
1991. Mutational Analysis of Hepatitis B Surface Antigen Particle Assembly and
Secretion. J Virol; 65: 3813-3820.
Bruss, V. and Vieluf, K.
1995. Functions of the Internal pre-S Domain of the Large Surface Protein in
Hepatitis B Virus Particle Morphogenesis. J Virol; 69: 6652-6657.
Budkowska, A., Bedossa,
P., Groh, F., Louise, A. and Pillot, J. 1995. Fibronectin of Human Liver Sinusoids
Binds Hepatitis B Virus: Identification by an Anti-Idiotypic Antibody Bearing
the Internal Image of the Pre-S2 Domain. J Virol; 69: 840-848.
Cheng, K.C., Smith, L.
and Moss, B. 1986. Hepatitis B Virus Large Surface Protein Is Not Secreted But
Is Immunogenic When Selectively Expressed by Recombinant Vaccinia Virus. J Virol;
60: 337-344.
Eble, B.E., Lingappa, V.R.
and Ganem, D. 1986. Hepatitis B Surface Antigen: An Unusual Secreted Protein
Initially Synthesized as a Transmembrane Polypeptide. Mol and Cell Biology;
6: 1454-1463.
Franco, A., Paroli, M.,
Testa, U., Benvenuto, R., Peschle, C., Balsano, F. and Barnaba, V. 1992. Tranferrin
Receptor Mediates Uptake and Presentation of Hepatitis B Envelope Antigen by
T Lymphocytes. J Exper Med; 175: 1195-1205.
Guo, J-T and Pugh, J.C.
1996. Topology of the Large Envelope Protein of Duck Hepatitis B Virus Suggests
a Mechanism for Membrane Translocation During Particle Morphogenesis. J Virol;
71: 1107-1114.
Heermann, K.H., Goldmann,
U., Schwartz, W. Seyffarth, T., Baumgarten, H. and Gerlich, W.H. 1984. Large
Surface Proteins of Hepatitis B Virus Containing the Pre-S Sequence. J Virol;
52: 396-402.
Heermann, K.H. and Gerlich,
W.S. 1991. Surface Proteins of Hepatitis B Viruses. In: McLachlan, A. (ed.)
Molecular Biology of the Hepatitis B Viruses. CRC Press, Boca Raton, pp. 109-144.
Le Bouvier, G.L., McCollum,
R.W., Hierholzer, W.J.J., Irwin, G.R., Krugman, S. and Giles, J.P. 1972. Subtypes
of Australia Antigen and Hepatitis B Virus. J American Medical Association;
222: 928-930.
Mehdi, H., Kaplan, M.J.,
Anlar, F.Y., Yang, X., Bayer, R., Sutherland, K. and Peeples, M.E. 1994. Hepatitis
B Virus Surface Antigen Binds to Apolipoprotein H. J Virol; 68: 2415-2424.
Mehdi, H., Yang, X. and
Peeples, M.E. 1996. An Altered Form of Apolipoprotein H Binds Hepatitis B Virus
Surface Antigen Most Efficiently. Virology; 217: 58-66.
Mehta, A., Lu, X., Block,
T.M., Blumberg, B.S. and Dwek, R. 1997. Hepatitis B Virus Envelope Glycoproteins
Vary Drastically in their Sensitivity to Glycan Processing: Evidence that Alteration
of a Single N-Linked Glycosylation Site Can Regulate HBV Secretion. Proc Natl
Acad Sci USA; 94: 1822-1827.
Melegari, M., Scaglioni,
P.P. and Wands, J.R. 1997. The Small Envelope Protein Is Required for Secretion
of a Naturally Occuring Hepatitis B Virus Mutant with Pre-S1 Deleted. J Virol;
71: 5449-5454.
Nagaraju, K., Naik, S.R.
and Naik, S. 1997. Functional Implications of Hepatitis B Surface Antigen (HBsAg)
in the T Cells of Chronic HBV Carriers. J Viral Hepat; 4(4): 221-230.
Neurath, A.R., Strick,
N. and Sproul, P. 1992. Search for Hepatitis B Virus Cell Receptors Reveals
Binding Sites for Interleukin 6 on the Virus Envelope Protein. J Experimental
Medicine; 175: 461-469.
Norder, H., Hammas, B.,
Lofdahl, S., Courouse, A.M. and Magnius, L.O. 1992. Comparison of the Amino
Acid Sequences of Nine Different Serotypes of Hepatitis B Surface Antigen and
Genomic Classification of the Corresponding Hepatitis B Virus Strains. J Gen
Virol; 73: 1201-1208.
Okamoto, H., Tsuda, F.,
Sakugawa, H., Sastrosoewignjo, R.I., Imai, M., Miyakawa, Y. and Mayumi, M. 1988.
Typing Hepatitis B Virus by Homology in Nucleotide Sequence: Comparison of Surface
Antigen Subtypes. J Gen Virol; 69: 2575-2583.
Persing, D.H., Varmus,
H.E. and Ganem, D. 1987. The preS1 Protein of Hepatitis B Virus Is Acylated
at Its Amino Terminus with Myristic Acid. J Virol; 61: 1672-1677.
Peterson, D.L. 1981. Isolation
and Characterization of the Major Protein and Glycoprotein of Hepatitis B Surface
Antigen. J Biological Chem; 256: 6975-6983.
Peterson, D.L., Paul, D.A.,
Lam, J., Tribby, I.I. and Achord, D.T. 1984. Antigenic Structure of Hepatitis
B Surface Antigen: Identification of the "d" Subtype Determinant by
Chemical Modification and Use of Monoclonal Antibodies. J Immuno; 132: 920-927.
Poisson, F., Severac, A.,
Hourious, C., Goudeau, A. and Roingeard, P. 1997. Both Pre-S1 and S Domains
of Hepatitis B Virus Envelope Proteins Interact with the Core Particle. Virology;
228: 115-120.
Pollicino, T, Zanetti,
A.R., Cacciola, I, Petit, M.A. Smedile, A., Campo, S., Sagliocca, L., Pasquiali,
M., Tanzi, E., Longo, G. and Raimondo, G. 1997. Pre-S2 Defective Hepatitis B
Virus Infection in Patients with Fulminant Hepatitis. Hepatology; 26(2): 495-499.
Pontisso, P, Ruvoletto,
M.G., Gerlich, W.H., Heermann, K-H, Bardini, R. and Alberti, A. 1989. Identification
of an Attachment Site for Human Liver Plasma Membranes on Hepatitis B Virus
Particles. Virology; 173: 522-530.
Stibbe, W. and Gerlich,
W.H. 1983. Structural Relationships Between Minor and Major Proteins of Hepatitis
B Surface Antigen. J Virol; 46: 626-629.
Stirk, H.J., Thorton, J.M.
and Howard, C.R. 1992. A Topological Model for Hepatitis B Surface Antigen.
Intervirology; 33: 148-158.
Swameye, I. and Schaller,
H. 1997. Dual Topology of the Large Envelope Protein of Duck Hepatitis B Virus:
Determinants Preventing Pre-S Translocation and Glycosylation. J Virol; 71:
9434-9441.
Werr, M. and Prange, R.
1997. Role for Calnexin and N-Linked Glycosylation in the Assembly and Secretion
of Hepatitis B Virus Middle Envelope Protein Particles. J Virol; 72: 778-782.
Xu, Z., Jensen, G. and
Yen, T.S. 1997. Activation of Hepatitis B Virus S Promoter by the Viral Large
Surface Protein via Induction of Stress in the Endoplasmic Reticulum. J Virol;
71(10): 7387-7392.
Xu, Z., Bruss, V. and Yen,
T.S.B. 1997. Formation of Intracellular Particles by Hepatitis B Virus Large
Surface Protein. J Virol; 71: 5487-5494.
Genomic
Map | Life Cycle | Particle
Types
Core/e Proteins | Surface
Proteins | Polymerase Protein | X
Protein
- Copyright ©
Robert G. 1997-2000 -






