Hepatitis B Polymerase Protein
The largest ORF in the HBV genome encodes for the hepatitis B polymerase protein (HBp). The protein is 90 kd in size and has RNA and DNA dependant polymerase activity. (1) As discussed earlier in this chapter, HBp plays a key role in HBV genome generation as well as pgRNA encapsidation. HBp is packaged together with pgRNA within HBV nucleocapsids. (2)
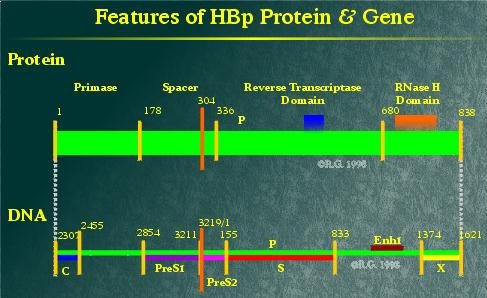
HBp has been divided into four characterized domains. Based on sequence homologies and studies on the mechanism of viral genome replication, most parts of HBp are indispensable. The N-terminus portion of the protein acts in priming (-)DNA strand synthesis and ends up covalently linked to the 5' end of the (-) DNA strand. This domain is termed primase.
The subsequent domain does not appear to have any enzymatic function, but acts as a spacer between the first and third domains.
The third domain gives HBp its name. It occupies approximately 40% of the protein and encodes for the RNA and DNA dependent polymerase activity. (3) However, HBp also requires the presence of metal ions and the presence of the -stem loop for polymerase/reverse transcriptase activity to occur. (4), (5), (6)
The fourth domain of HBp possesses its RNase H activity. (7), (8) This domain also plays a key role in HBV genome replication.
References:
1.
Toh, H., Hayashida, H. and Miyata, T. 1983. Sequency Homology Between
Retroviral Reverse Transcriptase and Putative Polymerases of Hepatitis
B Virus and Cauliflower Mosaic Virus. Nature; 305: 827-829.
2. Mack, D.H., Bloch, W., Nath, N. and Sninsky, J.J. 1988. Hepatitis B Virus Particles Contain a Polypeptide Encoded by the Largest Open Reading Frame: A Putative Reverse Transcriptase. J. Virol.; 62: 4786-4790.
3. Lanford, R.E., Kim, Y-H, Lee, H., Notvall, L and Beames, B. 1999. Mapping of the Hepatitis B Virus Reverse Transcriptase TP and RT Domains by Transcomplementation for Nucelotide Priming and by Protein-Protein Interaction. J. Virol.; 73: 1885-1893.
4. Urban, M., McMillan, D.J., Canning, G., Newell, A., Brown, E., Mills, J.S. and Jupp, R. 1998. In vitro Activity of Hepatitis B Virus Polymerase: Requirement for Distinct Metal Ions and the Viral Epsilon Stem-Loop. J. Gen. Virol.; 79: 1121-1131.
5. Bartenschlager, R. and Schaller, H. 1992. Hepadnaviral Assembly is Initiated by Polymerase Binding to the Encapsidation Signal in the Viral RNA Genome. EMBO; 7:4185-4192.
6. Tavis, J.E., Massey, B., and Gong, Y. 1998. The Duck Hepatitis B Virus Polymerase is Activated by its RNA Packaging Signal, Epsilon. J. Virol.; 72: 5789-5796.
7. Chang, L-J et al., 1990. Mechanism of translation of the hepadnaviral polymerase (P) gene. Proc. Natl. Acad. Sci. USA; 87: 5158-5162.
8. Radziwill, G. et al., 1990. Mutational analysis of the hepatitis B virus P gene product: domain structure and Rnase H activity. J. Virol; 64: 613-620.
